Appeals Court Backs Abortion Pill Restrictions; Supreme Court Appeal Planned
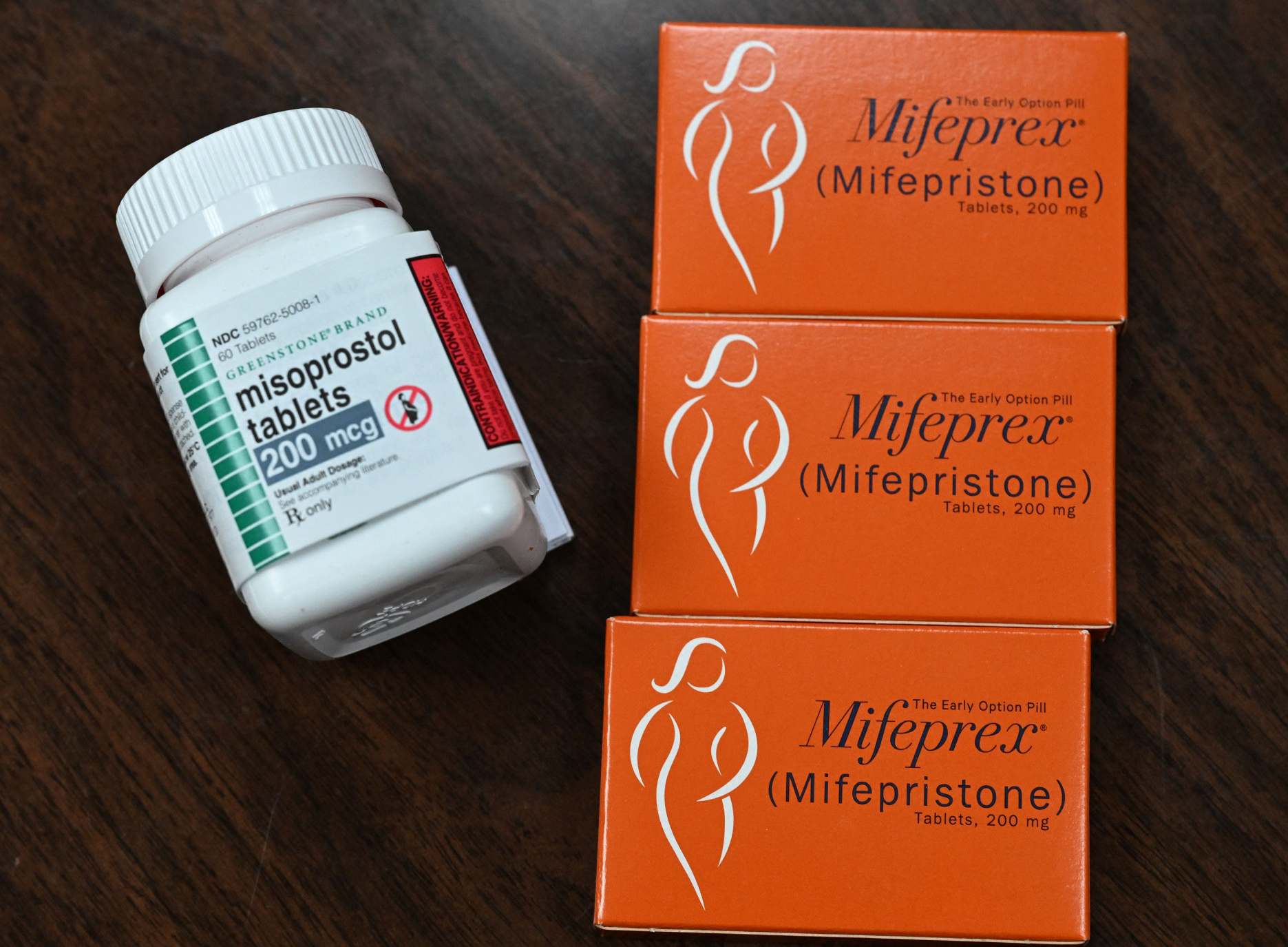
A U.S. court of appeals ruled Wednesday that access to the abortion drug mifepristone should be restricted. The ruling also banned telemedicine prescriptions as well as shipments by mail. However, the decision won’t take immediate effect.
The 5th U.S. Circuit Court of Appeals, based in New Orleans, did not rule that the drug must be taken off the market. The 5th U.S. Circuit Court of Appeals, based in New Orleans, did not rule that the drug should be taken off the market completely as a lower-court had done.
A spokesperson from the U.S. Department of Justice confirmed that the Biden Administration will appeal the decision to the U.S. Supreme Court. The Democratic President Joe Biden supports abortion rights. He ordered last year that the federal health agency expand access to mifepristone.
The Supreme Court will review the ruling in its next term, from October to June.
|
The five-judge panel of the 5th Circuit was reviewing an April order by U.S. District Court judge Matthew Kacsmaryk, in Amarillo Texas. Kacsmaryk stated that although it was a temporary ruling, he would likely make it permanent.
The ruling is the result of a lawsuit filed by four antiabortion groups, led by the newly formed Alliance for Hippocratic Medicine. Four antiabortion doctors also sued in November.
The FDA is accused of using an incorrect process to approve mifepristone, and not considering the safety of the drug when used by minors.
The 5th Circuit correctly required the FDA do its job, restore vital safeguards for women, girls and boys, including ending illegal abortions by mail order, Erin Hawley, a lawyer representing the anti-abortion organizations challenging the pill’s approval said in a press release.
Susan B. Anthony Pro Life America echoed this view in a press release, where it said that the FDA was “reckless.”
Alexis McGill Johnson is president of Planned Parenthood Federation of America. She said that the decision “makes clear that the FDA’s independent is still very much at risk as well as mifepristone approval.”
GenBioPro Inc., which sells a mifepristone generic, issued a statement by CEO Evan Masingill. “We remain worried about extremists and vested interests using courts to undermine science and the access to evidence-based medications, as well attempts to undermine U.S. Food and Drug Administration regulatory authority.”
CONSERVATIVE JUDGES
All three of the judges are conservatives who have a long history of opposing abortion. Circuit Judge James Ho said that he would go further and pull mifepristone from the market. The other two judges, however, felt the lawsuit was too late to contest the initial 2000 approval.
The majority of the panel reversed FDA actions which had made it easier to obtain the drug in recent years.
The court also reversed the agency’s 2016 decision to allow mifepristone to be used up to 10 weeks of pregnancy, rather than seven. The court reversed the agency’s 2016 decision allowing mifepristone use up to 10 weeks, instead of seven.
In her majority opinion, Circuit Judge Jennifer Walker Elrod stated that these steps were “taken without adequate consideration of the effect those changes would have upon patients.”
Last year, the U.S. Supreme Court overturned Roe v. Wade, a landmark ruling that legalized abortion in all 50 states.
According to Guttmacher Institute a research group that advocates abortion rights, since then at least 15 out of 50 states have prohibited abortions outright. Many others restrict it after a specific length of pregnancy.
Mifepristone and misoprostol are used in a medication abortion regimen that accounts for more than 50% of abortions in the United States.
Many medical studies and years of actual use have shown that the drug is effective and safe.
The American Medical Association and the American College of Obstetricians and Gynecologists, among others, claim that removing mifepristone from the market will harm patients because it would force them to undergo more invasive surgery abortions.









No Comments